It is possible to build rules so that cases are copied to so-called ‘Copy queues’. These are similar to ordinary validation queues in that cases and their interpretations are presented to the user, and if the interpretation for a case is changed, the case sent to the Knowledge Builder to trigger corrective rule building. However, there are key differences:
- Cases are never sent from a copy queue to the Online Information System. If a case has its interpretation changed, it is sent to the Knowledge Builder. If not, it is simply deleted after review.
- Copy queues can only be defined using rules in the Knowledge Builder.
- Cases cannot be posted between normal review queues and copy queues using the referral system.
A typical use of copy queues would be to allow an independent review of some or all cases at a site. The reviewer might not be associated with that site, and the review might be happening long after the cases were first processed by RippleDown, so it would not be appropriate for the reviewed cases to be sent to the Online Information System.
In order to review a copy queue, a user must have ‘Post-validation’ privileges, as defined in the Administrator. Conversely, a user with post-validation privileges is barred access from normal review queues unless they also have such privileges. A user may of course have both regular- and post-validation privileges, in which case they see all queues:
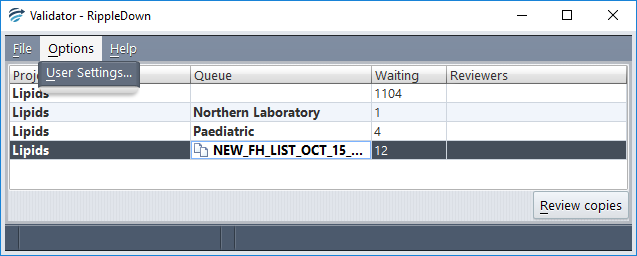
In order that a user with access to both review queues and copy queues does not inadvertently review one type of queue when they meant to review the other, copy queues are distinguished in two ways. In the Validator main screen, as shown above, copy queues have a special icon and are listed in green (which changes to red if selected). Further, in the case viewer screen, a ‘copy’ badge is shown as a watermark in the top right of the case view on all cases from a copy queue (see below):
Copy queues are only available for clinical projects, not Auditor ones.
To see how to build rules to add copy queues, read this help page.
